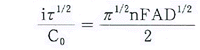| Microgravity
Experiments |  |

-
In microgravity, physical phenomena which never appear on
Earth may be clarified and the material constants may be measured more precisely.
This is because the space environment is totally different from the Earth.
For example, there is no liquid convection induced by the difference of
density of two materials, and two different materials with differing densities
can easily be mixed. In one space utilization application, new semiconductor,
composite material or new protein crystal are expected to be processed in
space. Industrial applications are urgently being sought.


Diffusion of Liquid metals and Alloys
Dr.Toshio Itami, Hokkaido University
1.Objectives of the Experiments
- Although the diffusion coefficient in a liquid state of pure metals and
alloys is industrially and scientifically important, few tests have been
performed with high accuracy on the ground because the effects of convection
are not negligible due to samples' high density and low viscosity. The Self
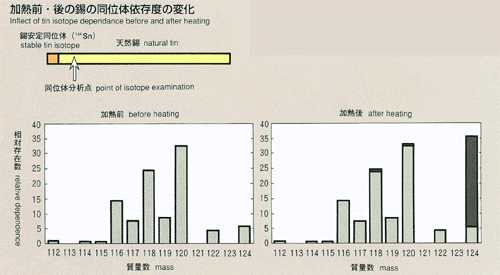
diffusion coefficient of tin, an easily handled metal, will be measured
using a stable isotope as a "tracer" of atomic transport. Tin
has a comparatively low melting point (231degrees), allowing a wide range
of temperature and precise examinations of temperature dependence. It is
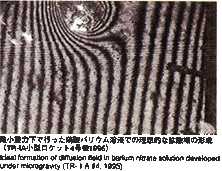
reported from the previous experiment performed on orbit that diffusion
coefficient of tin was 20 persent less than that measured on the ground
and the temperature coefficient is also different. In MSL-1, the temperature
range is widened and new technology is introduced in order to perform experiments
with higher accuracy. Results from these experiments should help us better
understand the mechanism of diffusion process in liquid metal.
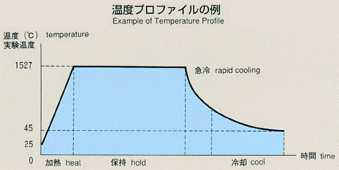
2.Method of Microgravity Experiment
- The most common method for measuring the diffusion coefficient, called
the long capillary method, is introduced for this experiment. With this
method the diffusion coefficient may be measured by examining time-dependent
variations of concentration of tracer which is initially placed in a capillary
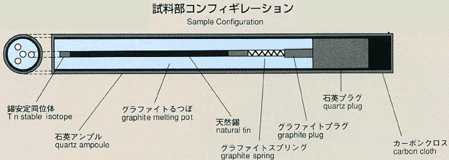
shaped sample. For MSL-1, a rod-shaped sample of tin (2mm diamiter, 60mm
long) with tin isotope (124Sn) placed on one end as a tracer is used. A
melting pot with four cylindrical holes, each with diameter of 2mm and length
of 80mm, made of graphite will be used. Also, a graphite lid and spring
are implemented to eliminate the existence of free surface in response to
the volume change of sample during heating, fusion, cooling and solidification
processes. To prevent oxidation of the sample, the melting pot is double
contained in cylindrical quartz and tantalum. After the sample is heated
and melted, the temperature will be held, allowing the diffusion to proceed
for a certain time. The sample will be cooled down and be solidified. The
diffusion coefficient will be determined by analyzing the isotope density
of the sample. Because the theoretical diffusion model is based on a constant
temperature, the diffusion which occurs during the heating and cooling process
introduces some errors into calculations. Rapid heating and cooling will
minimize these effects, and an accurate diffusion coefficient will be determined,
and compared with results from the Shear Cell Method.
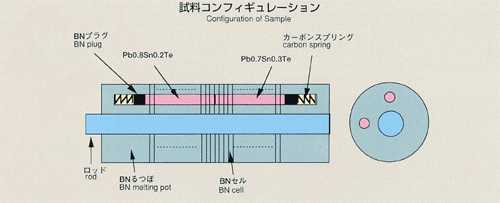
Diffusion in Liquid Lead-Tin-Telluride Ms.Misako Uchida, Ishikawajima-Harima
Heavy Industries
Ms.Misako Uchida, Ishikawajima-Harima Heavy Industries
1.0bjectives
- Diffusion coefficient is of great importance in manufacturing high-quality,
homogeneous crystals for semiconductors and must be considered to produce
quality devices, especially for combined semiconductors. In MSL-1, a combined
semiconductor, lead-tin-telluride, generally used for infrared semiconductor
lasers or exposures, will be provided and the diffusion coefficient of lead
and tin will be measured. The results should be of great use for determining
the best conditions to form a homogeneous crystal. From basic science aspects,
multiple atoms in molten combined semiconductors travel under influence
of the chemical bonding between them. An accurate diffusion coefficient
measured in space should help us better understand the mechanism of diffusion
in molten semiconductors.
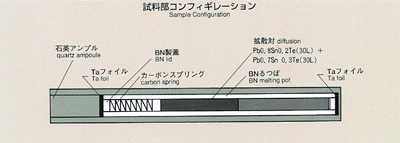
2.Method
- The most common method for measuring the diffusion coefficient, the long-capillary
method, is introduced for this experiment. With this method, the diffusion
coefficient may be measured by examining time-dependent variations of concentration
of tracer which is initially placed in a capillary-shaped sample. For MSL-1,
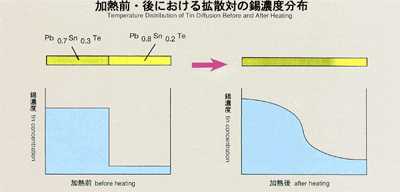
lead-tin-telluride formed of two different composition (Pb0.8Sn0.2Te and
Pb0.7Sn0.3Te) is used as a sample. Lead or tin may be used as a tracer.
A graphite melting pot with cylindrical holes, 2mm diameter, 80mm long,
will be used. Also, a graphite lid and spring are implemented to eliminate
the free surface in response to the sample volume change during heating,
fusion, cooling and solidification process. To prevent oxidation of the
sample, the melting pot is double-contained by a cylindrical quartz and
tantalum. After the sample is heated and melted, the temperature will be
held, allowing the diffusion to proceed for a certain time. The sample will
then be cooled down and solidified. The diffusion coefficient will be determined
by analyzing the isotope density of the sample. Because the theoretical
diffusion model is based on a constant temperature, the diffusion which
occurs during heating and cooling introduces some calculation errors. Rapid
heating and cooling will minimize these effects. An accurate diffusion coefficient
will then be determined, and compared with results from the Shear Cell Method.
Impurity Diffusion in Ionic Melts
Dr. Tsutomu Yamamura,
Tohoku University
1.Objectives
- Ionic melts is a molten chloride, consisting of an anion and a cation.
Studying diffusion in ionic melts should provide us with more information
and help us better understand the nature of electrical interactions. In
this research, the diffusion coefficient of ionic silver in ionic melts
(LiCl-KCl) will be measured by chronopotentiometry, a commonly used method.
This method requires only a brief period, and should be free of gravity-induced
convection. However, those experiments done on the ground have shown some
enormous range of results, implying the effect of gravity. In MSL-1, in
a microgravity environment that generates little gravity-induced convection,
an accurate diffusion coefficient will be measured in real time.
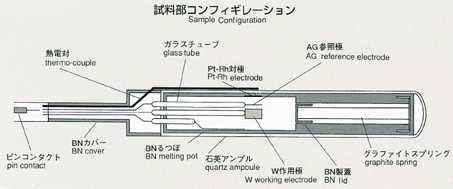
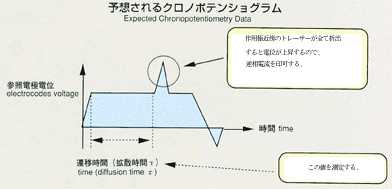
2.Method
- Applying a voltage between electrodes, inserted in ionic melts, induces
current. The degree of voltage or current depends on the numbers of ions,
travelling towards electrodes. Under certain condition, electric current
may be induced only by ionic diffusion.This will be suitable for measurement
by the electrochemical method. With chronopotentiometry, an appropriate
current is applied between electrodes. The diffusion difference coefficient
will be determined by analyzing the time variation of voltage difference
between electrodes. This voltage difference will maintain a constant value
in response to only diffusion of ions, which may be correlated by the following
equation. The diffusion coefficient will be calculated by inserting transfer
into the equation. The accuracy may be improved by applying low current
and by extending the transfer time. While ground experiments use higher
current and measure voltage faster to prevent the effect of gravity, space
experiments can use lower current in various levels and measure various
transfer times in order to obtain a diffusion coefficient with high accuracy.
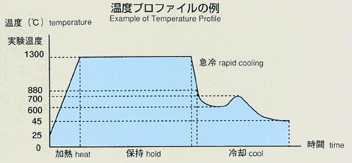
Lithium chloride and potassium are used with silver chloride added as a
tracer, and include electrodes for chronopotentiometry. The samples are
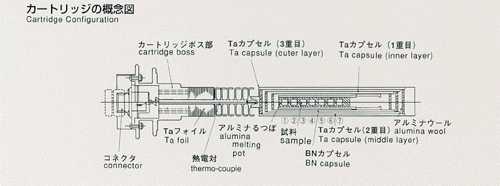
triple-contained (BN-made cylinderCquartz ampouleCtantalum cartridge,from
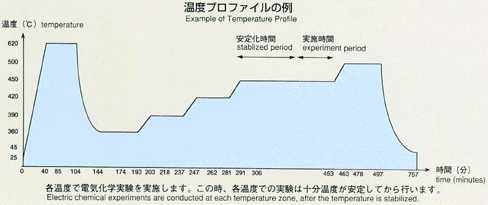
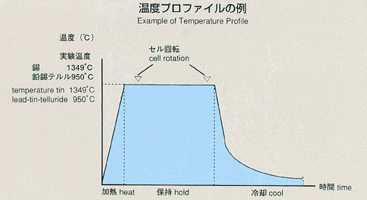
inner to outer). A standard temperature profile is shown below, and parameters
will be changed to cover various types of experiments. Chronopotentiometry
data, obtained through processing of samples, will be sent to the ground
for analysis by researchers.
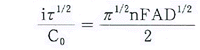
-
| Ñ | : | Transition
Time |
| Co | : | Initial Concentration |
| n | : | Transfar of electrons |
| A | : | Area of working electorode |
| D | : | Diffusion Coefficient |
| F | : | Faraday
constant |
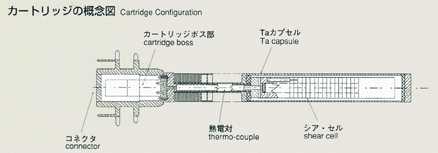
Measurement of Diffusion Coefficient by Shear Cell Method
Dr.Shinichi Yoda, National Space Development Agency of Japan(NASDA)
1.0bjectives
- An improved long-capillary, shear cell method will be introduced for the
diffusion experiment in MSL-1, and its reliability and mechanism will be
evaluated. Also, the same samples will be processed by the shear cell method
under identical conditions as those experiments using the long-capillary
method, and will be compared and studied together.
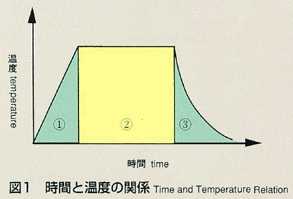
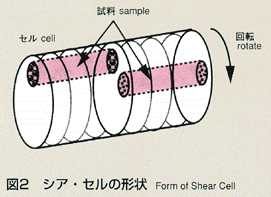
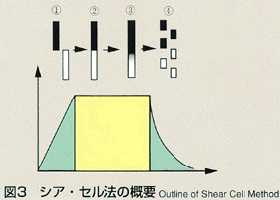
2.Method
- The shear cell method was conceived to make up for the disadvantage of
the long capillary method. The shear cell melting pot is constructed by
circular segments with a hole, and is capable of engaging and disengaging
the molten samples by rotating the cells. In the shear cell method, the
molten samples will be engaged only while temperature is constant, minimizing
the effect of diffusion during heating and cooling. This method also has
the advantage that it can accurately measure samples which have a large
volume expansion and contraction, or a great difference in melting point.
The disadvantage of the shear cell method is that rotation of cells may
disturb the process of diffusion due to the inertia or viscosity of samples.
The thickness of cell segments and rotation rate, which are critical to
the accuracy, are analyzed and optimized prior to the manufacturing of shear
cells. As a result, the thickness is determined to be 2mm and 28 cell segments
are to be used to construct a melting pot for high accuracy. Two samples,
lead and lead-tin-telluride, will be used, inserted in BN-made melting pots,
and contained in tantalum-made cartridges. The samples will be heated up
to the target temperature and engaged by shear cell rotation done manually
by crew members on orbit. After holding for some duration, the crew will
rotate the cells again to disengage the samples and terminate the diffusion
process. The density distribution of tracer will be examined post mission
using Secondary Ion Mass Spectroscopy (SIMS) and Electron Probe Micro Analyzer
(EPMA) to determine the diffusion coefficient and effects of cell rotation
during the process.
Liquid Phase Sintering
Dr.Randall German, Pennsylvania State University
1.Objectives
-
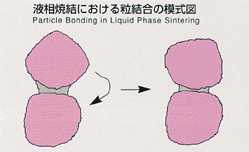
Sintering is usually used to form materials which have a
high melting point. However, this method requires high temperature and long
duration for heating in order to bond the junctions between ground material
particles by congelation or diffusion. Liquid phase sintering, employed
in MSL-1, makes it possible to process the sintering at lower temperature
and short duration by saturating molten metals, which have lower melting
points, into ground materials. At the junctions between powdered materials
which have high melting points, the effect of interface force is dominant,
promoting the local process of melting and eduction, and bonding between
particles will be accelerated. The existence of high-density particles and
the reaction against gravity disturb particle formation and melt circulation,
resulting in uniform sintering. The mechanism of sintering with respect
to gravity has not been clarified. This experiment is expected to clarify
the mechanism of liquid phase sintering, succeeding from lML-2.
2.Method
- The samples, powdered tungsten, nickel and iron, with seven different
compositions are individually contained in tantalum cartridges and will
be heated up to 1500degrees. Experiments will be performed with various
holding duration of heating.
Diffusion Process in Molten Semiconductors
Dr.David N.Matthiesen, Case Western Reserve University
1.Objectives
- Various types of semiconductors can be made by adding some other elements
to silicon or germanium. The diffusion coefficient of impure elements in
semiconductors is of great importance and critical to manufacturing processes
of good quality. A definitive measurement of diffusion coefficient will
explain the mechanism of diffusion, and high quality semiconductors can
be manufactured. However, an exact diffusion coefficient has not been derived
from ground-based experiments due to the effect of gravity. The samples
of germanium and gallium, with tin and antimony added as impurities, are
used and the diffusion coefficient will be measured using shear cell method
under two different temperature conditions, isothermal and thermogradient.
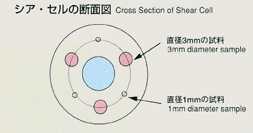
Also, the effect of the melting pot wall will be investigated by varying
the diameter of samples.
2.Method
- ln MSL-1, NASA-made shear cells will be used to measure the diffusion
coefficient accurately. The mechanism of shear cells is generally the same
as that of the one NASDA developed, which is capable of disengaging and
engaging the samples by rotating the cell segments. The samples with diameter
of 1mm and 3mm are used. The samples will be heated up to the target temperature
and engaged by shear cell rotation done manually by crew members on orbit.
After holding for some duration, the crew will rotate the cells aging to
disengage the samples and the diffusion process will be terminated. The
impurity density will be analyzed, and the diffusion coefficient will be
determined after the mission.
Last Updated : February 18, 1998
![[HOME]](home.gif)
![[INDEX]](index.gif)
![[TOP]](top.gif)