This is an archive of information released in the past.
Disclaimer: It may contain broken links or outdated information. Some parts may not function in current web browsers.
*Visit https://humans-in-space.jaxa.jp/en/ for the latest information.

Experiment
- News
- Kibo Utilization Strategy
- Kibo Utilization Plan
- List of JAXA's Utilization Themes
- Experiment Facilities
- Space Environment Utilization
- Archive
Background and Objectives of the experiment
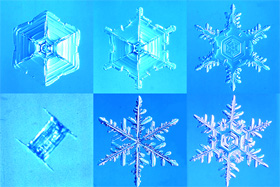
Figure 1. Crystal Pattern (Snow)
(Image credit: Prof. Yoshinori Furukawa, Hokkaido University)
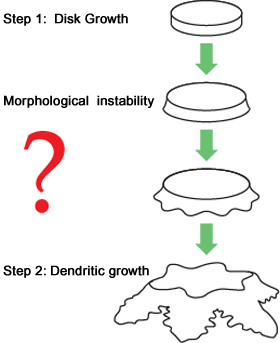
Figure 2. Formation process of an ice crystal
You must have seen, at least once, snow on your sweater, or water vapor frozen on your windows.
You can see that those are beautiful hexagonal crystals with several components of slightly different branch-like shapes assembled, or crystals with components of the same shape equally spaced.
This variety of shape of the crystal patterns is due to slight difference of physical conditions during snow/ice crystal formation which includes, temperature, humidity, wind speed, the amount of dust (Figure 1).
The formation process of ice crystals (the crystal growth process) is complex, and the detailed formation mechanism remains unknown.
The process starts with formation of a disc-shaped crystal.
As the crystal formation progress, the disc-shaped crystal becomes unstable at its edge and grows to be a dendritic crystal called a dendrite (Figure 2).
Previous studies revealed the growth process of dendrites to a certain extent, including the mechanism of branching.
But it is unknown how a smooth, disc-shaped crystal becomes unstable, that is, how the instability at the edge starts to grow.
To examine the process, a stable experimental environment without any flow of water is needed for the precise measurement of temperature distribution of water surrounding the crystal and its temporal change.
Convection of water or air can be observed when you boil water, or you turn on a heater in your room. It is called “heat convection”.
The heat convection of a liquid such as water or air is caused by gravity when the density of the liquid is changed by heat.
Such heat convection is unavoidable on Earth. The latent heat from a growing ice crystal causes heat convection, which results in instability of physical conditions around the ice crystal.
Thanks to the microgravity environment, the heat convection won’t occur in the International Space Station.
Therefore, it is necessary to perform space experiments for more precise observation of detailed destabilization process of the disc-shaped crystal.
Outline of the Experiment

Figure 3. The external view of the Ice Crystal Cell

Figure 4. Inside of the Ice Crystal Cell

Figure 5. A diagram of the cell cartridge of the Ice Crystal test specimen
Observing from two directions enables us to understand the morphology of the crystal in three dimensions.

Figure 6. A disc-shaped ice crystal growing from the tip of a glass capillary
Left: Image taken by an Amplitude-modulation microscopy
Right: Image taken by a Mach-Zehnder (MZ) Interference Microscope
The stripe pattern around the crystal is distorted when the temperature changes due to latent heat.
The ambient temperature of the crystal can be calculated by examining distortion of the pattern in this image.
This experiment uses the Solution Crystallization Observation Facility (SCOF).
The SCOF is equipped with microscopes and an interferometer so that it can examine the crystal growth processes in detail.
Figure 3 and 4 show a test specimen (Note1) used for this experiment. It is called “Ice Crystal Cell”. Figure 5 shows a diagram of a sample cell inside the test specimen.
The ”Ice Crystal Cell” is installed in the SCOF.
When the nucleation cell is cooled, the ice crystal nucleates in the nucleation cell.
The ice grows into the crystal growth cell through the glass capillary.
The process of ice crystal formation in the temperature-controlled crystal growth cell, especially the step in which the instability of a disc-shaped crystal grows, will be observed in detail, to measure thickness, diameter and growth speed of the ice crystal.
And the local temperature around the crystal will also be examined usingthe interferometer (Figure 6).
The experiments will be conducted repeatedly to check the reproducibility of the measurements. under different temperature conditions in the crystal growth cell.
”Ice Crystal Cell” is equipped with an observation system so that the crystal growth process can be observed from two directions.
The observation system of the SCOF gives a plain view of the growing crystal while the one of the ”Ice Crystal Cell” gives a side view, so that the crystal's morphology and its surrounding temperature can be analyzed in three dimensions.
The mystery of the formation of ice crystals, which are very familiar to us, will be solved by this space experiment.
Note1: “The test specimen” is a small experimental apparatus which has been developed for each experiment.
 |
Principal Investigator Yoshinori FURUKAWA Professor, Institute of Low Temperature Science, Hokkaido University |
 |
| Copyright 2007 Japan Aerospace Exploration Agency | Site Policy |