This is an archive of information released in the past.
Disclaimer: It may contain broken links or outdated information. Some parts may not function in current web browsers.
*Visit https://humans-in-space.jaxa.jp/en/ for the latest information.

Benefits for Humanity
- Benefits for Humanity
- Program Science Forum
- International Partners
High-Quality Protein Crystal Growth Experiment Onboard "Kibo"
Mika Masaki
Space Environment Utilization Center, JAXA
The vast universe and proteins used to form our bodies are not as unrelated as they may sound -a "crystal" is the key to connect the two. Proteins in the body have three-dimensional, complex structures and the most suitable environment to study these structures is space. Recent studies in Japan, based on the International Space Station research, may help develop more effective methods for treating disease.
In space, there is no convection causing solution to flow up and down and right to left due to differences of density, nor is there precipitation to cause heavier items to sink. Therefore, protein molecules form orderly and create a high quality crystal that is beneficial to the study of its structure. Various crystals have been created in the unique environment of space.
The Japan Aerospace Exploration Agency, or JAXA, has conducted nine sessions of the protein crystallization experiments since 2003 in the Zvezda service module on the International Space Station and has established a technology to produce high-quality protein crystals in space. Based on the technology, a series of six experiments conducted in Kibo began in July 2009, and completion is expected, by the beginning of 2013.
With the support of Russian Federal Space Agency known as Roscosmos, the protein samples installed in the Cell Units (Fig. 1) are launched to the space station on board the Russian Progress spacecraft. Soon after the docking, the samples are brought into Kibo to be placed inside the Protein Crystallization Research Facility, or PCRF, where they will be kept for a period of two to four months at a stable temperature, 68 degrees Fahrenheit (20 degrees Celsius) (Fig. 2). A counter-diffusion method called “Gel-Tube method,” is used for crystallization whereby polyethylene glycol or salt solution is diffused into the protein solution separated by a porous membrane inside a tube. Then, concentration of polyethylene glycol in the protein solution gradually increases and finally satisfies the condition for protein crystallization (Fig. 3).

Fig.1 Cell Unit
Cell Unit is used for incubation of the proteins when it is installed into the Protein Crystallization Research Facility or PCRF on board the space station. It is also a transportation container of the Gel-Tube cartridges as it is loaded into the cargo vehicles.

Fig.2 Protein Crystallization Research Facility
Protein Crystallization Research Facility, or PCRF, is a sub-rack payload used for the protein crystallization experiments, which provides controlled temperature and can hold six cell units (up to 144 proteins) inside.
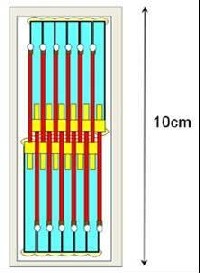
Fig.3 Gel-Tube Cartridge (JCB)
Gel-Tube cartridge called “JCB” which contains twelve crystallization capillaries inside is a simple and economical crystallization tool. It requires only minimum onboard operation of astronauts.
A major purpose of the protein crystal growth experiment is its contribution to the development of medical treatments. The protein that causes disease and the medicine that suppresses it can be compared to the relation between a “keyhole” and a “key.” If the shape of the keyhole is discovered by examining the structure of the protein, treatment-oriented medicine with few side effects - the key to fit the keyhole - can be designed. JAXA is making positive advancements in their research on obstinate diseases through experiments in space with the hope of supporting medical care more effectively (Fig. 4).
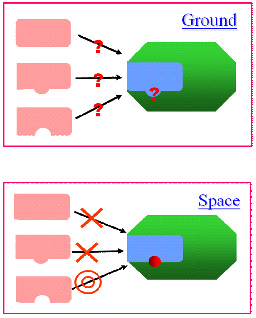
Figure 4. Advantage of the Space Experiment
Because the structure of the disease-causing protein, or the keyhole, is vague when it is obtained on the ground, the shape of the key, or a medicine candidate compound for treatment, cannot be determined. However, it is possible to find the structure of the disease-causing protein through the space experiments, and medicine that fits the treatment (the key that fits the keyhole) can be developed. (クA・JAXA)
An example of a protein that was successfully crystallized in space that may hold the key to treating a disease is hematopoietic prostaglandin D synthase or H-PGDS. H-PGDS is the enzyme responsible for the production of hematopoietic prostaglandin D synthase 2 or PGD2, which is known to be a mediator of allergic and inflammatory reactions.
Recently, a research team at the Osaka Bioscience Institute or OBI reported that H-PGDS is expressed in necrotic muscle fibers of patients with Duchenne muscular dystrophy or DMD. An inherited muscle disorder, DMD is the most common form of muscular dystrophy, affecting approximately 1 in 3,500 boys. DMD causes muscular atrophy and accelerates the progression of muscular deterioration. It is an obstinate disease for which a fundamental mode of treatment has not yet been found. Therefore, H-PGDS-specific inhibitors are considered to be useful drugs for muscular dystrophy.
The OBI research team has successfully determined the three-dimensional structure of H-PGDS in a complex with a prototype H PGDS-specific inhibitor. H-PGDS has been crystallized several times in microgravity as part of JAXA’s space experiments. Using X-ray crystallographic analysis - using X-rays to determine the structure - researchers determined the structure of the high-quality crystals of H-PGDS-inhibitor complexes diffracting to 1.0 - 1.5 Angstrom resolution in space, and discovered a new inhibitor with several hundred times stronger activity than the prototype inhibitor (Fig. 5). This provides a better understanding of the binding mode of H-PGDS with inhibitors for better drug design. Further research is ongoing with an aim of practical use (Fig. 6).
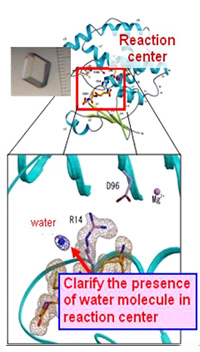
Figure 5. High-Quality Crystals of H-PGDS-Inhibitor Complexes
The detailed structure of muscular dystrophy related protein became clear through a space experiment. (クA・Osaka Bioscience Institute/MARUWA Foods and Biosciences, Inc.)
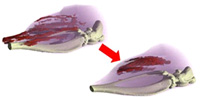
Figure 6. Treatment for Muscular Dystrophy
The progress of muscular atrophy is reduced by an effective medicine candidate compound. (クA・Osaka Bioscience Institute)
There are more than 100,000 proteins in the human body and as many as 10 billion in nature. Every structure is different and each one of them holds important information related to our health and to the global environment. Space is now being utilized as the latest site of biomedical research, where scientists conduct experiments that are impossible or extremely difficult on Earth to understand the structures of proteins. Various investigations in Kibo generate high-quality protein crystals open doors to new possibilities. The elucidation of protein structure is instrumental to understanding the mechanisms of life.
| Copyright 2007 Japan Aerospace Exploration Agency | Site Policy |







